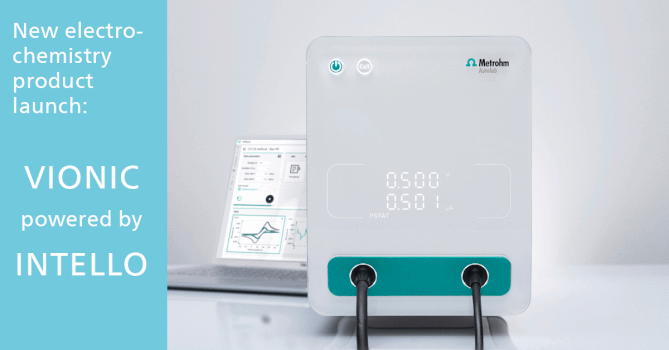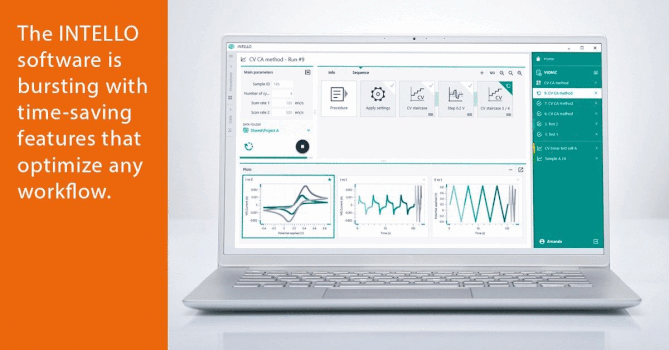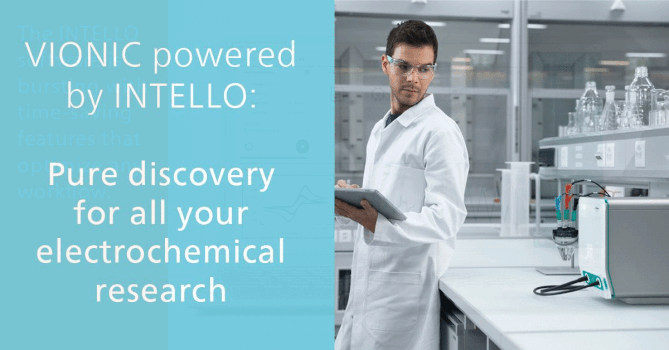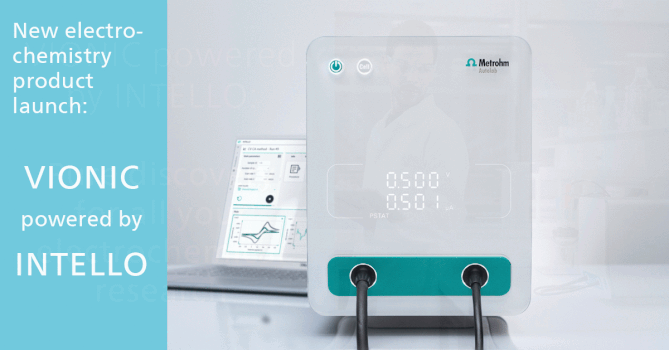
Dissolution Study in the Evaluation of Oral Preparations with Controlled Drug Release
Keywords:
dissolution test, oral dosage forms, simulation of digestive tract conditionsAbstract
Dissolution studies bring important characteristics of the oral preparations. They are used for estimation of behavior of new dosage forms in vivo. Pharmacopoeias prescribe dissolution testing using buffers, which can contain enzymes and surfactants. However, current methods are not always mimicking the real conditions in vivo. The preparations administered orally pass through the gastrointestinal tract (GIT) at varying pH values. A dissolution method was developed intended for drug targeting into the colon using the time periods and pH of buffers corresponding to those in the GIT. The dissolution method in the presence or absence of β-glucosidase was used to evaluate drug release from pellets coated with a polysaccharide or polyacrylic.